Publications
Thunder-DDA-PASEF enables high-coverage immunopeptidomics and identifies HLA class-I presented SarsCov-2 spike protein epitopes
David Gomez-Zepeda, Danielle Arnold-Schild, Julian Beyrle, Elena Kumm, Ute Distler, Hansjörg Schild, Stefan Tenzer
 privat
privat
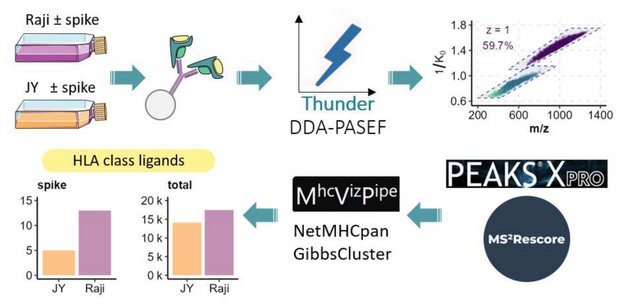
Human leukocyte antigen (HLA) class I peptide ligands (HLAIps) are key targets for developing vaccines and immunotherapies against infectious pathogens or cancer cells. Identifying HLAIps is challenging due to their high diversity, low abundance, and patient-specific profiles. Here, we developed a highly sensitive method for identifying HLAIps using liquid chromatography-ion mobility-tandem mass spectrometry (LC-IMS-MS/MS). The optimized method, Thunder-DDA-PASEF, semi-selectively fragments HLAIps based on their IMS and m/z, thus increasing the coverage of immunopeptidomics analyses. Thunder-DDA-PASEF includes singly-charged peptides, which contributes to more than 35% of the HLAIp identifications. Combined with MS2Rescore, Thunder-DDA-PASEF improved ligandome coverage by 150% compared to the original-DDA-PASEF method, and enabled in-depth profiling of HLAIps from two human cell lines, JY and Raji, transfected to express the SARS-CoV-2 spike protein. We identified seventeen spike protein HLAIps, thirteen of which had been reported to elicit immune responses in human patients.
Metabolic regulation of immune responses to cancer
Jannis Wißfeld*, Anke Werner*, Xin Yan*, Nora Ten Bosch*, Guoliang Cui
*authors contributed equally
 CMB/J.Wißfeld
CMB/J.Wißfeld
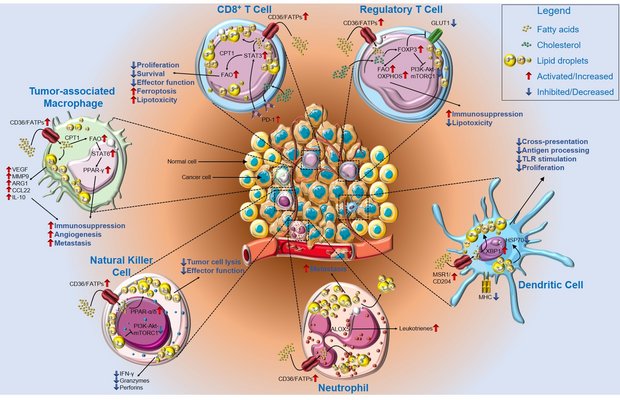
The tumor microenvironment is an ecosystem composed of multiple types of cells, such as tumor cells, immune cells, and cancer-associated fibroblasts. Cancer cells grow faster than non-cancerous cells and consume larger amounts of nutrients. The rapid growth characteristic of cancer cells fundamentally alters nutrient availability in the tumor microenvironment and results in reprogramming of immune cell metabolic pathways. Accumulating evidence suggests that cellular metabolism of nutrients, such as lipids and amino acids, beyond being essential to meet the bioenergetic and biosynthetic demands of immune cells, also regulates a broad spectrum of cellular signal transduction, and influences immune cell survival, differentiation, and anti-tumor effector function. The cancer immunometabolism research field is rapidly evolving, and exciting new discoveries are reported in high-profile journals nearly weekly. Therefore, all new findings in this field cannot be summarized within this short review. Instead, this review is intended to provide a brief introduction to this rapidly developing research field, with a focus on the metabolism of two classes of important nutrients-lipids and amino acids-in immune cells. We highlight recent research on the roles of lipids and amino acids in regulating the metabolic fitness and immunological functions of T cells, macrophages, and natural killer cells in the tumor microenvironment. Furthermore, we discuss the possibility of "editing" metabolic pathways in immune cells to act synergistically with currently available immunotherapies in enhancing anti-tumor immune responses.



